noble gas configuration for iodine|ground state electron configuration iodine : Manila Gas (predicted) Position in Periodic table: Group: 18, Period: 7, Block: p: Category: . Lokasyon: Philippines
PH0 · noble gas notation iodine
PH1 · noble gas core configuration
PH2 · noble gas configuration worksheet
PH3 · noble gas configuration chart
PH4 · noble gas abbreviation sulfur
PH5 · noble gas abbreviation
PH6 · ground state electron configuration iodine
PH7 · full electron configuration of iodine
PH8 · Iba pa
By creating an account, you agree to our Terms & Conditions and confirm that you are at least 18 years old or over and all information given is true.
noble gas configuration for iodine*******Mar 23, 2023
Gas (predicted) Position in Periodic table: Group: 18, Period: 7, Block: p: Category: .
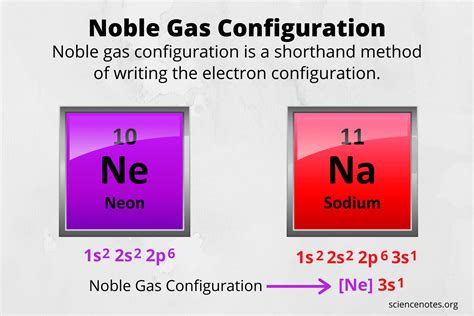
Learn how to write a noble gas configuration for any atom using the Aufbau principle and the periodic table. Find the noble gas core and the valence electron. A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the remaining electrons. So for sodium, we make the .
Using the Aufbau Principle, the Pauli Exclusion Principle, and Hund's rule to predict an atom's electron configuration using the periodic table as . The arrangement of electrons in iodine in specific rules in different orbits and orbitals is called the electron configuration of iodine. The electron configuration of iodine is 4d 10 5s 2 5p 5, if the electron .
In order to write the I electron configuration we first need to know the number of electrons for the I atom (there are 53 electrons). When we write the .
noble gas configuration for iodine ground state electron configuration iodine The electronic configuration notation of I is also written as [Kr] 4d10, 5s2, and 5p5, where 36 electrons come from the Krypton noble gas configuration, while the .

In the short notation, you place brackets around the preceding noble gas element followed by the valence shell electron configuration. The periodic table shows that kyrpton (Kr) is the .This video shows you how to write the ground state electron configuration using noble gas notation (abbreviation) for the elements fluorine, sulfur and cadmium.The noble gas configuration of iodine (I) is [Kr] 5s^2 4d^10 5p^5. This means that it has the same electron configuration as krypton (Kr) with additional electrons filling the 5s,.
Unless specified, use any method to solve the following problems. Answers are given in noble gas notation. 1. Find the electron configurations of the following: silicon; tin; lead; 2. Scenario: You are . Iodine electron configuration. The 4d orbital is now full. So, the remaining five electrons enter the 5p orbital. Therefore, the iodine complete electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 . The noble gas shorthand electron configuration for iodine in its ground state is [Kr]5s24105p5 . How do atoms achieve noble gas configurations in typical single covalent bonds? Atoms try to take on an electron configuration of a .Atoms form ions so as to achieve electron configurations similar to those of the noble gases. For the following pairs of noble gas configurations, give the formulas of two simple ionic compounds that would have comparable electron configurations. a. He an; If an atom had 7 electrons in its second shell, it would need to electron(s) to become .This video shows you how to write the ground state electron configuration using noble gas notation (abbreviation) for the elements fluorine, sulfur and cadmium. This is the shortcut or shorthand method of writing the electron configuration for elements. Iodine is a chemical element with symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a lustrous, purple-black non-metallic solid at standard conditions that melts to form a deep violet liquid at 114 degrees Celsius, and boils to a violet gas at 184 degrees Celsius. What is the electron configuration of iodine (I .
The electron configuration is the standard notation used to describe the electronic structure of an atom. Under the orbital approximation, we let each electron occupy an orbital, which can be solved by a single wavefunction. . (Kr) is the previous noble gas listed before iodine. The noble gas configuration encompases the energy .
noble gas configuration for iodine First, select the input. You can select the element name or atomic number if you want. Enter the element name or atomic number in the blank box based on your input selection. Click the Calculate button. After submission, the given element’s name, symbol, atomic number, atomic weight and noble gas electron configuration will also be shown.
The Philippine News Agency is a web-based newswire service of the Philippine government under the supervision of the News and Information Bureau (NIB) of the Presidential Communications Operations Office (PCOO).
noble gas configuration for iodine|ground state electron configuration iodine